Together with Dr. Hans Husby and my colleagues Prof. Roy Johnsen, Prof. Afrooz Barnoush, and Dr. Mariano A. Kappes we have recently published the first in a series of new original research papers focused on the influence of nickel on the hydrogen embrittlement resistance of low alloy steels (LAS). In this article, we concentrate on the effect of nickel in solid solution. Specifically, we quantified changes in hydrogen permeation as a function of the Ni content, keeping all other metallurgical and environmental factors constant.
Why is it important?
As we discussed before here and here, the restrictions imposed by ISO 15156–2 regarding the allowable nickel content remain controversial to date. In this work, Hans studied hydrogen diffusion kinetics as a function of nickel content in solid solution in the ferrite phase. Understanding how nickel influences hydrogen permeation is one of the building blocks to gaining a better understanding of hydrogen stress cracking performance.
Varying nickel while maintaining all other variables unchanged is not a simple task! Indeed, as explained by Hans, nickel affects the final microstructure both directly and indirectly, which makes separating the influence of other parameters challenging. We had to develop special heat treatment procedures to both eliminate banding and obtain similar grain sizes. Whereas banding is an artifact from the rolling operation, grain size is directly affected by nickel since Ni promotes grain refinement. Figure 1 illustrates the removal of the severe banding found in the as-received condition.
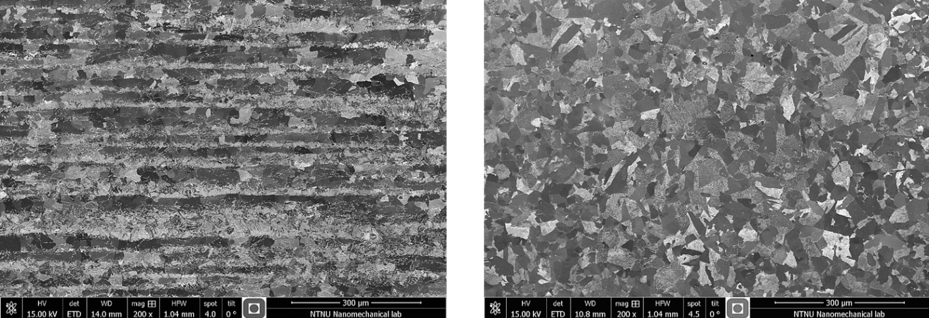
Figure 1. Micrograph normal to the rolling direction of a 2 wt% Ni sample in a) an as-received and b) heated to 930 °C followed by furnace cooling to 500 °C condition.
Abstract
Nickel offers several beneficial effects as an alloying element to low alloy steels. However, it is, in the oil and gas industry, limited by part 2 of the ISO 15156 standard to a maximum of 1 wt% due to sulfide stress cracking resistance concerns.
Hydrogen uptake, diffusion, and trapping were investigated in research-grade ferritic/pearlitic low alloy steels with Ni contents of 0, 1, 2 and 3 wt% by the electrochemical permeation method as a function of temperature and hydrogen charging conditions.
Qualitatively, the effective diffusion coefficient, Deff, decreased with increasing Ni content. The sub-surface lattice hydrogen concentration, C0, decreased with increasing Ni content in all charging conditions while the trend between the sub-surface hydrogen concentration in lattice and reversible trap sites, COR, and Ni content varied with the charging conditions. Irreversible trapping, evaluated by consecutive charging transients, was not observed for any of the materials. Lastly, the possible influence of an increasing fraction of pearlite with increasing Ni content is discussed.
Citation
H. Husby, M. Iannuzzi, R. Johnsen, M. Kappes, A. Barnoush, Effect of nickel on hydrogen permeation in ferritic/pearlitic low alloy steels. Int J Hydrogen Energ 43, 3845–3861 (2018). https://doi.org/10.1016/j.ijhydene.2017.12.174
H. Husby, M. Iannuzzi, R. Johnsen, M. Kappes, A. Barnoush, Effect of nickel on hydrogen permeation in ferritic/pearlitic low alloy steels. Int. J. Hydrogen Energ. 43, 3845-3861 (2018). doi: 10.1016/j.ijhydene.2017.12.17
You can download a copy here or by clicking on the figure above.