Aqueous carbon dioxide corrosion of carbon steel is an electrochemical process involving the anodic dissolution of iron and the cathodic evolution of hydrogen. The overall reaction is shown in Equation 1 (LaTex Code):
Example
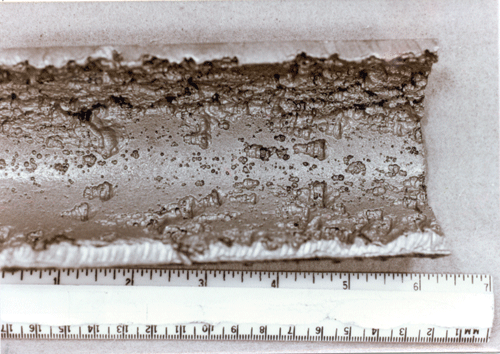
References
- S. Nešić, “Key issues related to modeling of internal corrosion of oil and gas pipelines – A review”, Corrosion Science 49, (2007): p.4308–4338.