In a previous post I mentioned we were working on a review paper focused on hydrogen embrittlement, in particular sulfide stress cracking (SSC), of nickel-containing low alloy steels (LAS). You may be wondering why nickel? What makes steels alloyed with nickel unique?
Nickel does not form carbides and, therefore, delays pro-eutectoid ferrite, pearlitic, and bainitic reactions 1. Transformation-temperature-time (TTT) curves can be used to illustrate the effect of alloying elements on retarding diffusion-assisted transformations. Figure 1 shows the effect of nickel on TTT curves by comparing nickel-free UNS G41400 (SAE/AISI 4140) steel vs. UNS G43400 (SAE/AISI 4340), a nickel-containing LAS of similar composition, Table 1.
Table 1. UNS G41400 (SAE/AISI 4140) and UNS G43400 (SAE/AISI 4340) nominal composition.
| Alloy (UNS) | C (wt%) | Mn (wt%) | Cr (wt%) | Mo (wt%) | Ni (wt%) |
|---|---|---|---|---|---|
| G41400 | 0.37 | 0.77 | 0.98 | 0.21 | – |
| G43400 | 0.42 | 0.78 | 0.80 | 0.33 | 1.79 |
As seen in Figure 1, a 1.79-wt% Ni addition delays the nose of the ferritic/pearlitic transformation by approximately 100 seconds 2. Likewise, nickel shifts the bainite nose by approximately 8 seconds. Why is this important? Upper bainite, which forms at elevated temperatures, is an undesirable microstructure due to its poor combination of strength and toughness, associated with the precipitation of carbides between ferrite laths 3. By delaying the bainite reaction, nickel additions result in steels with a lower bainitic microstructure. This is critical when heat treating heavy sections. Figure 1 also shows that upper bainite finish temperatures are displaced to longer times.
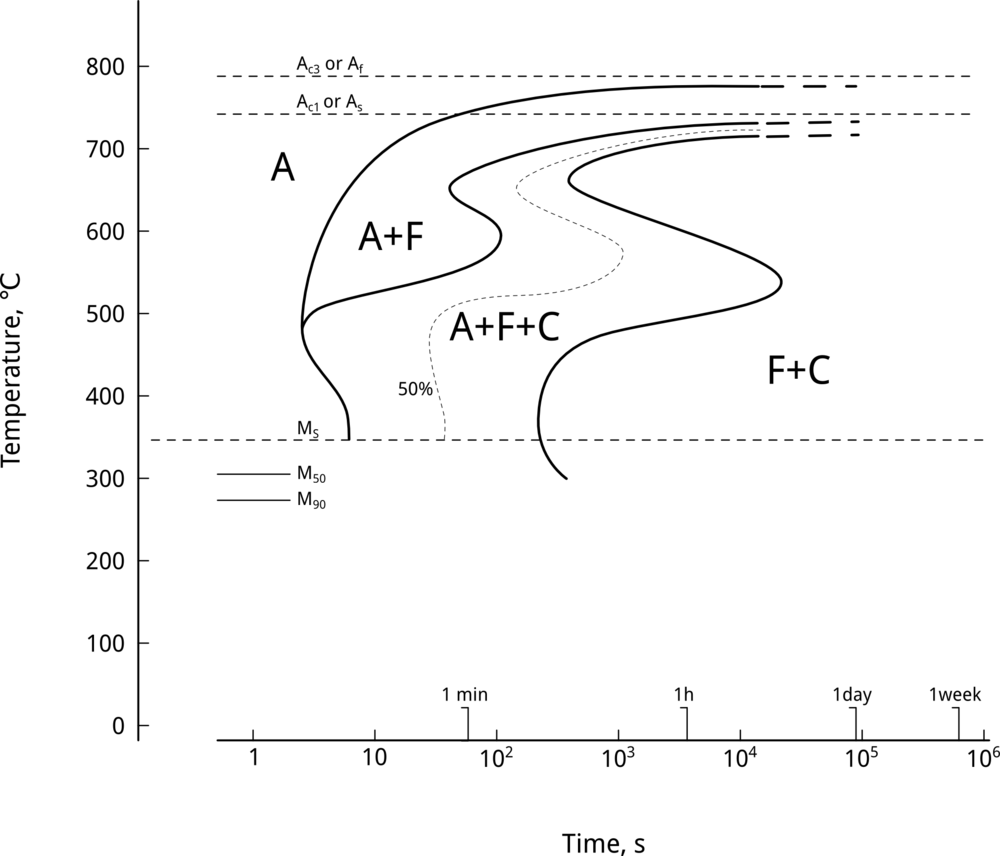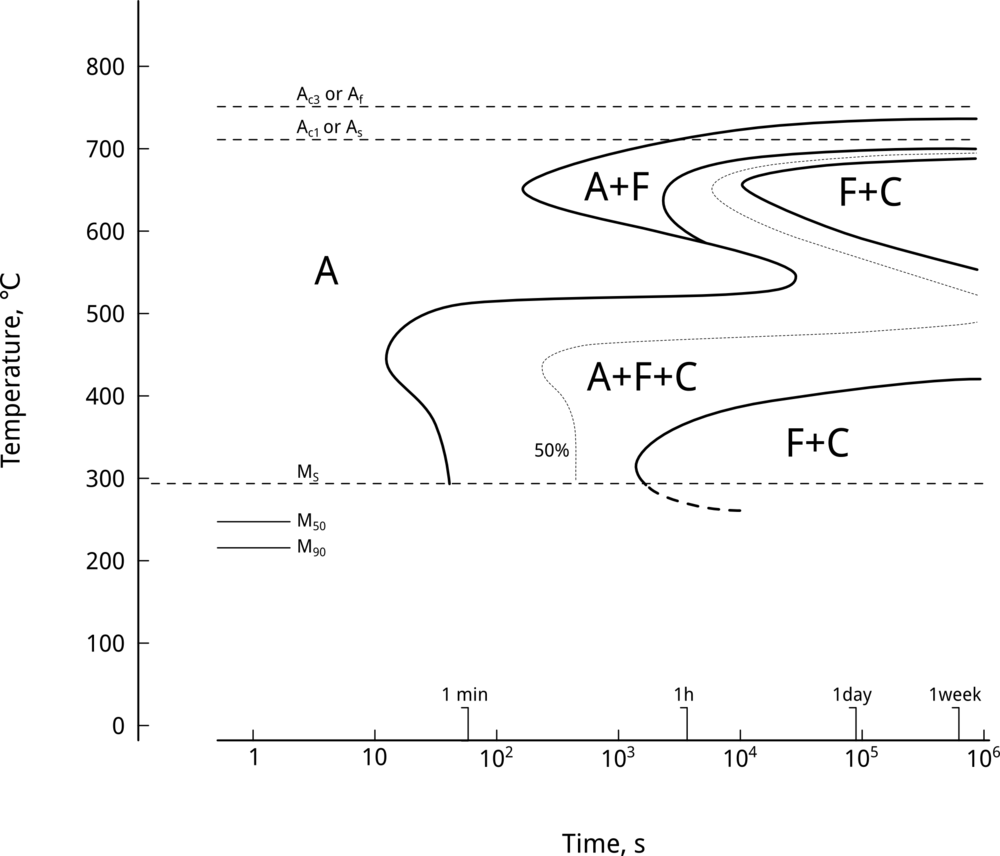
Nickel also delays the position of ferrite start and bainite start reactions on continuous cooling transformation (CCT) diagrams 4. A 1 wt% Ni addition to a Mo-Nb modified UNS G43400 (SAE/AISI 4340) steel resulted in an increase in ferrite start time of about 5000 s, whereas the bainite start time was displaced by about 300 s. As a result, nickel additions to low alloy steels improve hardenability, imposing, as we will see later, a very small penalty on weldability.
References
- C. Siebert, D. Doane, D. Breen, “The Hardenability of Steels: Concepts, Metallurgical Influences, and Industrial Applications”, ASM International, (Metals Park, OH, 1977). Buy at amazon ↩
- G.F. Vander Voort, “Atlas of Time-Temperature diagrams for iron and steels”, ASM International (Metals Park, OH, 1991). Buy at Amazon ↩
- G. Krauss, Chapter 6: Bainite, in: “Steels: Processing, Structure, and Performance”, 1st ed., ASM International, (Metals Park, OH, 2005): pp. 87–100. Buy at Amazon. ↩
- R. Garber, “Higher Hardenability Low Alloy Steels for H2S Resistant Oil Country Tubulars”, Corrosion 39, (1983): p.83–91. ↩